DISCOVERIES REPORTS (ISSN 2393249X), 2020, volume 3
CITATION:
Towhid ST, Rakhi NN, Arefin ASMS, Saha O, Mamun S, Moniruzzaman M, Rahaman MM. COVID-19 and the Cardiovascular System: How the First Post-Modern Pandemic ‘Weakened’ our Hearts. Discoveries Reports, 2020; 3: e15. DOI: 10.15190/drep.2020.9 Submitted: Nov. 03, 2020; Revised: Dec. 29, 2020; Accepted: Dec. 29, 2020; Published: Dec. 31, 2020;
Access FULL text of the manuscript here: Full text (PDF)
COVID-19 and the Cardiovascular System: How the First Post-Modern Pandemic ‘Weakened’ our Hearts
Syeda Tasneem Towhid (1, #), Nadira Naznin Rakhi (2, #), ASM Shamsul Arefin (3), Otun Saha (4), Sumaiya Mamun (5), Mohammad Moniruzzaman (6), Md. Mizanur Rahaman (4, *) (1) Department of Microbiology, Jagannath University, Dhaka, Bangladesh (2) Department of Biotechnology and Genetic Engineering, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalganj, Bangladesh (3) Department of Biomedical Physics and Technology, University of Dhaka, Dhaka 1000, Bangladesh (4) Department of Microbiology, University of Dhaka, Dhaka 1000, Bangladesh (5) Institute of food and Nutrition, University of Dhaka, Dhaka 1000, Bangladesh (6) Department of Biology, Virginia Polytechnic Institute and State University, Blacksburgh, VA 24060, USA
# Equal Contribution
*Correspondence to: Md. Mizanur Rahaman, Associate Professor, Department of Microbiology, University of Dhaka, Dhaka 1000; Email: razu002@du.ac.bd; Phone: +8801796585290.
Abstract
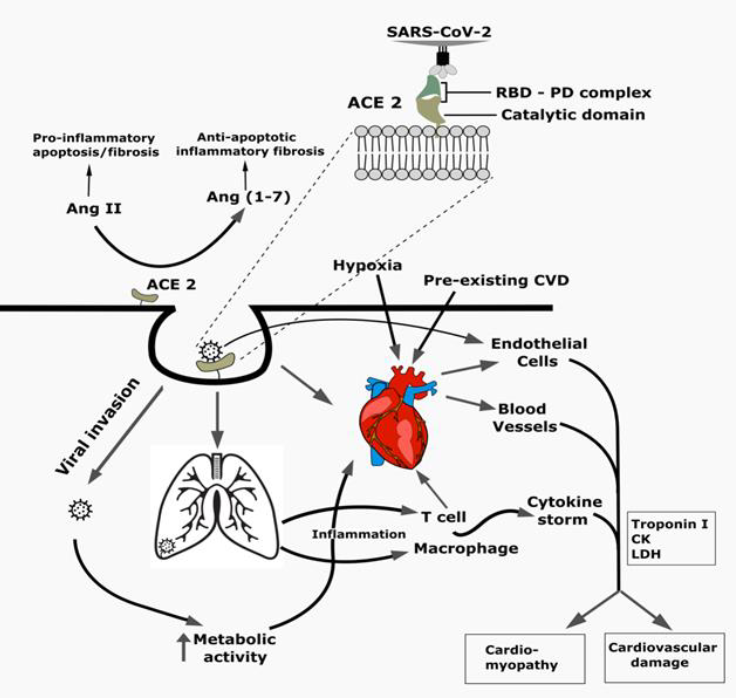
The global spread of SARS-CoV-2 with its diverse signs and symptoms manifested in COVID-19 patients across different age groups and geographic locations perplexed the clinicians and public health experts. Emerging variants of SARS-CoV-2 through continuous mutation with a limited arsenal of treatment made the study of viral pathogenesis and factors associated with disease outcomes in a holistic approach inevitable, among which pre-existing cardiovascular complications were found to be significantly associated with adverse outcome of COVID-19. In addition, COVID-19 has already been reported to cause cardiac injury and different cardiovascular complications in patients irrespective of preexisting cardiovascular complications, which highlights the importance of recognizing the complications at the onset, although these arising complications might be an indirect effect of SARS-CoV-2 induced cytokine storm or hypoxia rather the virus itself. Also, the drugs used for the clinical management of the patients may have an impact on the induced cardiac complications. Thus, the effect of SARS-CoV-2 on the cardiovascular system needs to be investigated in order to predict the clinical outcome and to devise a proper treatment strategy. Besides, the interaction of vaccines or therapeutics to be approved with the cardiovascular system needs to be evaluated to avoid confounding effects leading to cardiovascular complications followed by post-approval retraction. However, potential biomarkers (eg. troponin, D-dimers, fibrin) associated with cardiac injury may be potentially useful in predicting life-threatening conditions early enough to save lives. In conclusion, this review summarizes the molecular pathogenesis of cardiovascular damage caused by SARS-CoV-2 in COVID-19 patients, as well as prescribed treatment and preventative measures.
References
1. Mim MA, Rakhi NN, Saha O, Rahaman MM. Recommendation of fecal specimen for routine molecular detection of SARS-CoV-2 and for COVID-19 discharge criteria. Pathogens and global health.2020; 114(4):168-169.
2. Sahin AR. 2019 Novel Coronavirus (COVID-19) Outbreak: A Review of the Current Literature. Eurasian J Med Oncol. 2020; 4(1):1-7.
3. Worldometers.info. Worldometer COVID-19 Data. https://www.worldometers.info/coronavirus. Last accessed on December 25, 2020.
4. Islam MM, Rakhi NN, Islam OK, Saha O, Rahaman MM. Challenges to be considered to evaluate the COVID-19 preparedness and outcome in Bangladesh. Int J Healthc Manag. 2020; 1-2.
5. Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129-33.
6. Shi S, Qin M, Shen B, et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-10.
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020; 323(11):1061-69.
8. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (covid‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infec. J Am Heart Assoc. 2020; 9(7):e016219.
9. Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020; 368(6494): 1012-15.
10. Saha O, Rakhi NN, Towhid ST, Rahaman MM. Reactivation of Severe Acute Respiratory Coronavirus-2 (SARS-CoV-2): Hoax or hurdle? Int J Healthc Manag.2020;1-2.
11. Jin, Ying-Hui, Lin Cai, Zhen-Shun Cheng, Hong Cheng, Tong Deng, Yi-Pin Fan, Cheng Fang et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7(1): 4.
12. Hoffmann M, Kleine-Weber H, Krueger N, Mueller MA, Drosten C, Poehlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020.
13. South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020; 318(5): 1084-90.
14. Mason RJ.Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020; 55: 2000607.
15. Hoffmann M, Kleine-Weber H, Schroeder S et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020; 182(2):271-80.
16. Jeffers SA, Tusell SM, Gillim-Ross L et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U S A. 2004; 101(44): 15748-53.
17. Zhang S, Li H, Huang S, You W, Sun H. High-resolution CT features of 17 cases of Corona Virus Disease 2019 in Sichuan province, China. Eur Respir J. 2020;55(4): 2000334.
18. Xu Z, Shi L, Wang Y et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8(4):420-22.
19. Zhang L, Zhu F, Xie L et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020; 31(7):894-901.
20. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. Respir Med 2020; 31(7): 894-901.
21. World Health Organization. Q&A on coronaviruses (COVID-19). Published online 2020.
22. Liu Y, Yang Y, Zhang C et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364-74.
23. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46(5):846-48.
24. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020; 45(3):230-32.
25. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020;248.
26. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020; 116(6):1097-1100.
27. Tan W, Aboulhosn J.The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. 2020; 309(15): 70-77.
28. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020; 10(2): 102:08.
29. Varga Z, Flammer AJ, Steiger P et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395(10234): 1417-18.
30. Li B, Yang J, Zhao F et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020; 109(5): 531-38.
31. Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA. 2020; 323(13): 1239-42.
32. Saha O, Hossain MS, & Rahaman MM. Genomic exploration light on multiple origin with potential parsimony-informative sites of the severe acute respiratory syndrome coronavirus 2 in Bangladesh. Gene reports. 2020; 21:100951.
33. Klok FA, Kruip MJHA, van der Meer NJM et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020.
34. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020; 18(40:844-47.
35. Magro C, Mulvey JJ, Berlin D et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res.2020; 220:1-13.
36. Chu CM, Cheng VCC, Hung IFN et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax.2004;59(3):252-56.
37. Khan Z, Karataş Y, Rahman H. Anti COVID-19 Drugs: Need for More Clinical Evidence and Global Action. Adv Ther.2020: 37:2575-79.
38. Russell B, Moss C, Rigg A, Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: Should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020: 14(1023):1-3.
39. Williams B, Mancia G, Spiering W et al. ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypert. J Hypertens. 2018; 36(10):1953-2041.
40. Jessup JA, Gallagher PE, Averill DB, et al. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol - Hear Circ Physiol. 2006; 291(5): 2166-72.
41. Sodhi M, Etminan M. Safety of ibuprofen in patients with COVID-19: causal or confounded? Chest.2020; 158(1): 55-56.
42. Giollo A, Adami G, Gatti D, Idolazzi L, Rossini M. Coronavirus disease 19 (Covid-19) and non-steroidal anti-inflammatory drugs (NSAID). Ann Rheum Dis. 2020:0:1.
43. Meyerowitz EA, Vannier AGL, Friesen MGN et al. Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J. 2020; 34(5): 6027:37.
44. Şireli M, Saǧmanligil V, Çetin N, Emre B. Electrocardiographic changes induced by ivermectin in Guinea pigs. Scand J Lab Anim Sci. 2020; 35(1): 45-49.
45. Hossain MS, Hami I, Sawrav MSS, Rabbi MF, Saha O, Bahadur NM, Rahaman MM. Drug Repurposing for Prevention and Treatment of COVID-19: A Clinical Landscape. Discoveries. 2020; 8(4): e121. DOI: 10.15190/d.2020.18
46. US Food and Drug Administration. FDA Issues Emergency Use Authorization for Convalescent Plasma as Potential Promising COVID–19 Treatment, Another Achievement in Administration’s Fight Against Pandemic. Accessed on Decemeber 19, 2020; Available at: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment
47. Chai CK, Valk SJ, Piechotta V et al. Convalescnet Plasma or Hyper-immunoglobulin for people with COVID-19: A Living Systematic Review. Cochrane Database of Systematic Reviews. 2020; 10:1-4
48. Xi Y. Convalescent Plasma Therapy for COVID-19: A Tried and True Old Strategy. Signal Transduction and Targeted Therapy. 2020; 5:203
49. Zeng H, Wang D, Nie , et al. The Efficiency Assessment of Convalescent Plasma Therapy for COVID-19 Patients: A Multi-center Case Series. Signal Transduction and Targeted Therapy. 2020; 5:219
50. Devsenapathy N, Ye Z, Loeb M, Fang F, Najafabadi T. Efficiency and Safety of Convalescent Plasma for Severe Respiratory Virus Infections: A Systematic Review and Meta-Analysis. CMAJ.2020;192 (27):E745-55. doi: 10.1503/cmaj.200642
51. Duan K, Liu B, Li, et al. Effectiveness of Convalescnet Plasma Therapy in Severe COVID-19 Patients. PNAS. 2020; 117(17):9490-9496
52. Maor Y, Cohen D, Paran N, Israely T, Ezra V. Compassionate use of convalescent plasma for treatment of moderate and severe pneumonia in COVID-19 patients and association with IgG levels in donated plasma. EC Clinical Medicine. 2020; 26:100525
53. Abolgasemi H, Eshghi P, Cheraghal Am Et Al. Clinical Efficacy of Convalescent Plasma for Treatment Of Covid-19 Infections: Results of A Multicenter Clinical Study. Transfusion Apheresis Science. 2020; 59(5):102875.
54. Casadevall A, Joyner M, Pirofski LA. A randomized trial of Convalescent Plasma for COVID-19 Potentially Hopeful Signal. JAMA. 2020; 324(5):455-457.
55. Joyner JM, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin Proc. 2020 Sep; 95(9): 1888–1897.
56. Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711–20.
57. Clar C, Oseni Z, Flowers N, Keshtkar-Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev. 2015;(5):CD005050.
58. Astra Zeneca. ZD1222 Oxford Phase III trials interim analysis results published in The Lancet. Astra Zeneca. Accessed on December 20, 2020; Available at: https://www.astrazeneca.com/media-centre/press -releases/2020/azd1222-oxford-phase-iii-trials-interim-analysis-results-published-in-the-lancet.html
59. Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020; 396: 1595–606
60. Voysey M, Clemens SAC, Madhi SA et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2020; S0140-6736(20)32661-1.
61. Mullard A. COVID-19 Vaccine Development Pipeline Gears up. Lancet. 2020; 395.
62. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. New England Journal of Medicine. 2020; N Engl J Med 2020; 383:1920-1931.
63. Renn A, Fu Y, Hu X, Hall MD, Simenov A. Fruitful Neutralizing Antibody Pipeline Brings Hope To Defeat SARS-Cov-2 Trends in Pharmacological Sciences, 2020;41(11):815-830.
64. US Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine. December 11, 2020
65. Pfizer Pharmaceuticals. All COVID-19 Updates. Accessed on December 20, 2020. Available at: https://www.pfizer.com/health/coronavirus/updates
66. Sahin U, Muik A, Derhovanessian E et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020; 586: 594-612
67. Zhang Y, Zeng G, Pan H. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet. 2020; 1473-3099
68. Guo T, Fan Y, Chen M et al. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811-818.
69. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020; 58(7): 1131:1134.
70. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3): 390–391.
71. Vestjens SMT, Spoorenberg SMC, Rijkers GT et al. 2020. High-sensitivity cardiac troponin T predicts mortality after hospitalization for community-acquired pneumonia. Respirology.2017;22(5):1000-1006.
72. Yang J, Zheng Y, Gou X et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95.